Tumor cells exhibiting moderate (2+) to strong (3+) membranous staining3-5

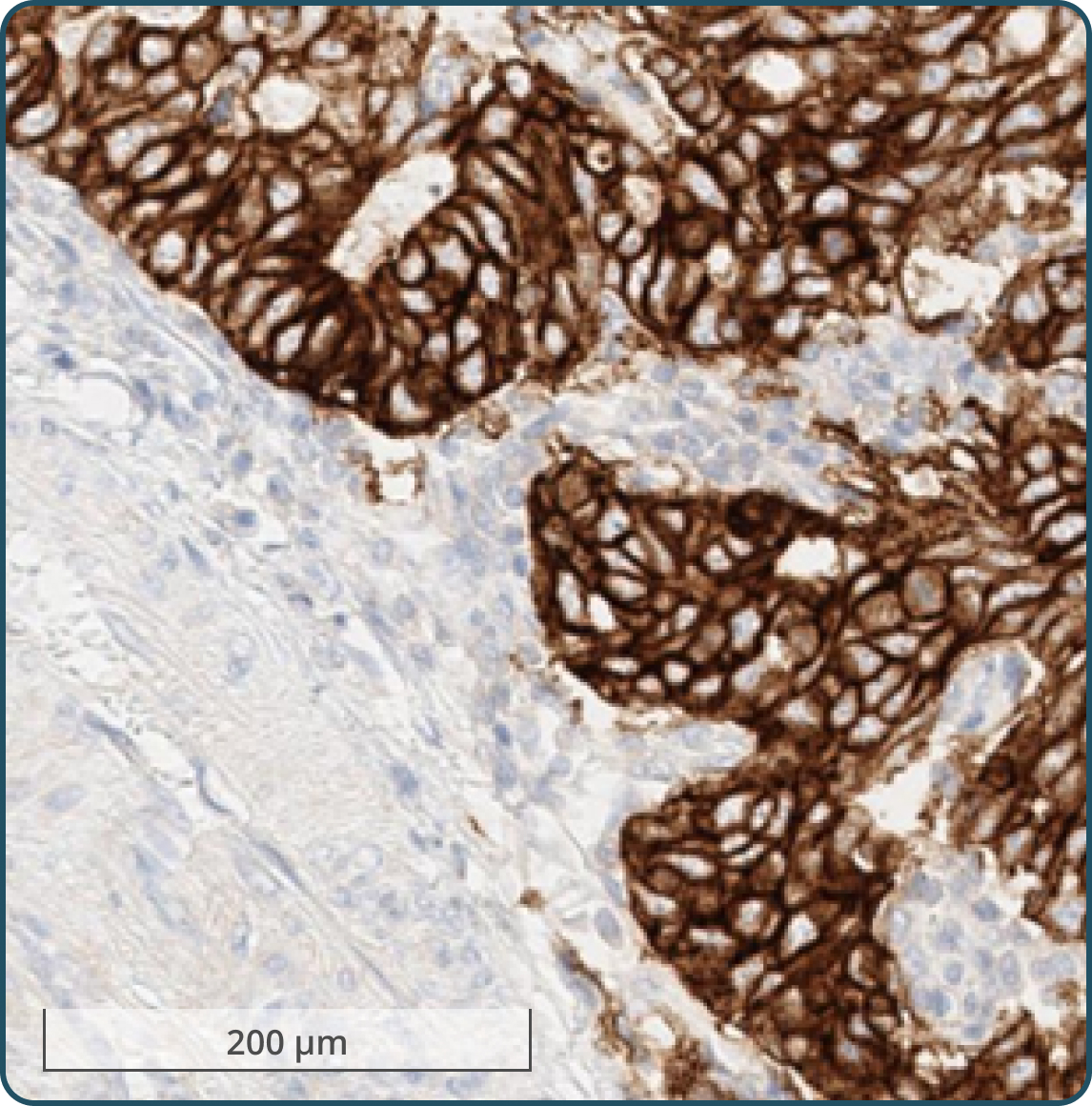
Any tumor cells with moderate (2+) to strong (3+) membranous staining, including any of the following:
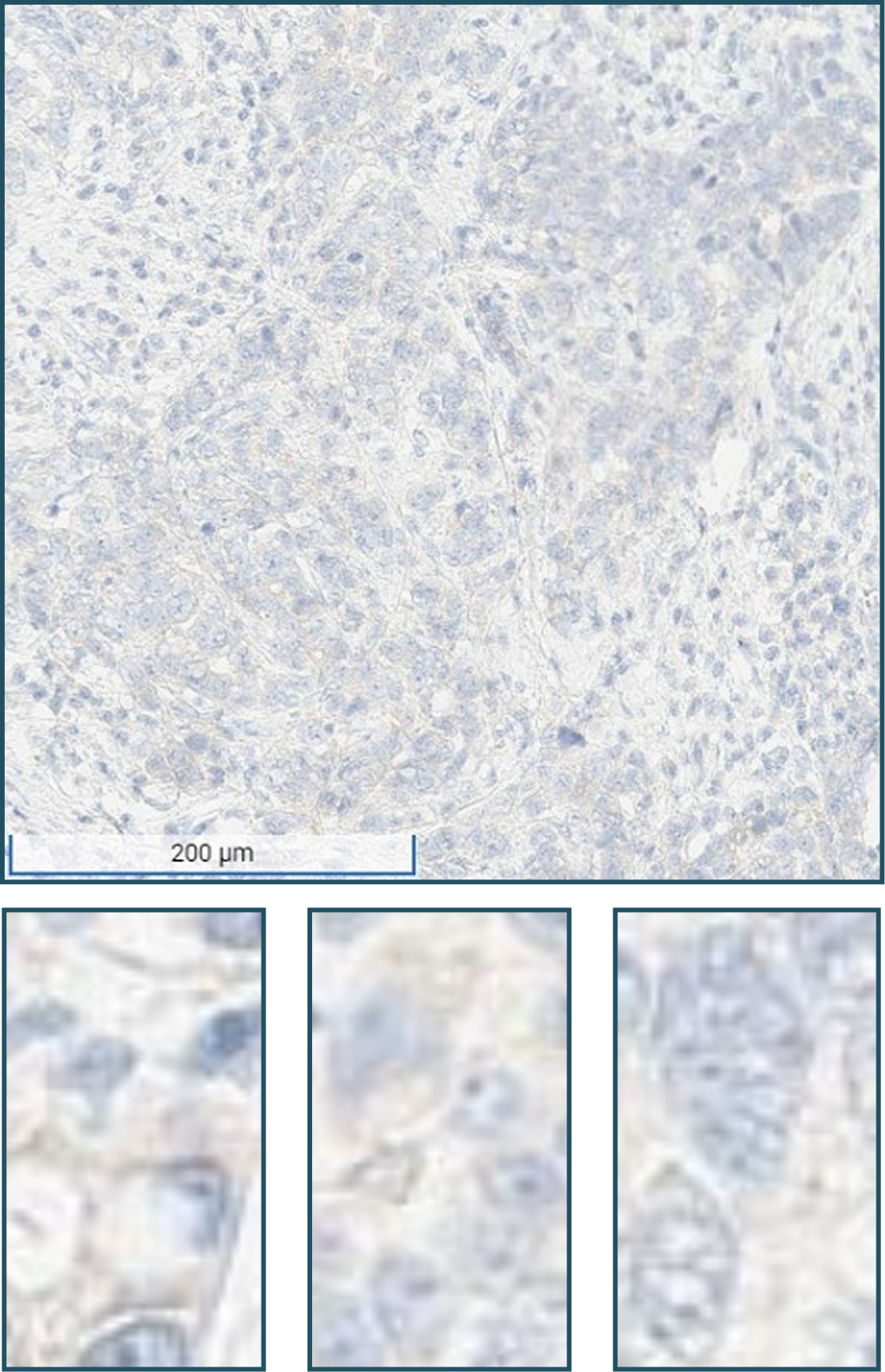
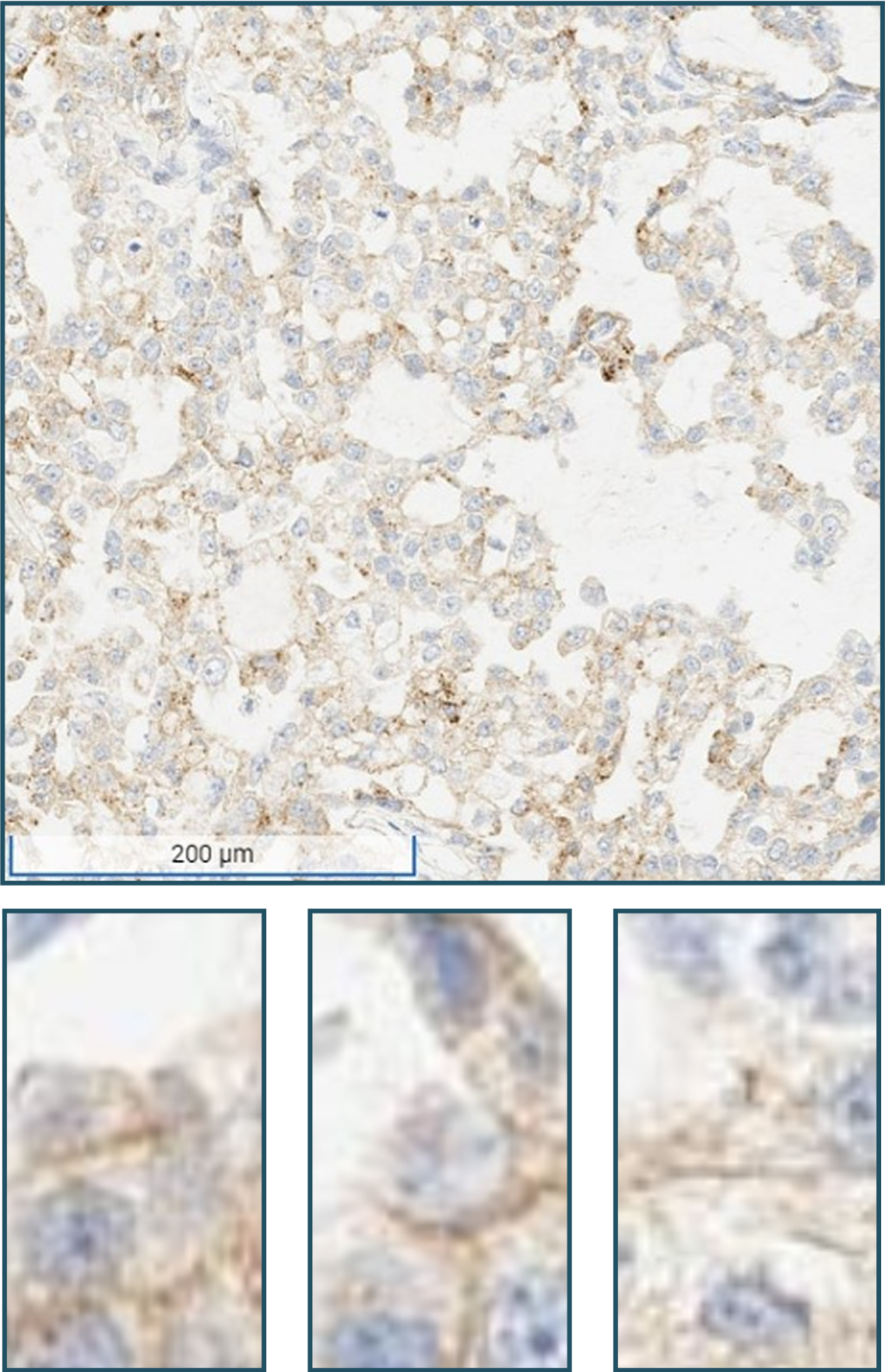
No tumor cells with moderate (2+) to strong (3+) membranous staining OR tumor cells only exhibiting:
*VENTANA FGFR2b (FPR2-D) Mouse Monoclonal Antibody is intended for laboratory use in the qualitative immunohistochemical detection of FGFR2b by light microscopy in sections of formalinfixed, paraffin-embedded tissues stained on a BenchMark IHC/ISH instrument. There is no approved therapy for FGFR2b.
FGFR2b, fibroblast growth factor receptor 2, isoform IIIb; GEJ, gastroesophageal junction; H&E, hematoxylin and eosin; IHC, immunohistochemistry.
References: 1. Rha SY, et al. JCO Precis Oncol. 2025;9:e2400710. 2. Data on File, Amgen; 2024. 3. Ahn S, et al. Mod Pathol. 2016;29:1095-1103. 4. Wainberg ZA, et al. Lancet Oncol. 2022;23:1430-1440. 5. Wainberg ZA, et al. Gastric Cancer. 2024;27:558-570. 6. Roche Diagnostics. https://elabdoc-prod.roche.com/eLD/api/downloads/65a1283e-a4f6-ee11-2591-005056a71a5d?countryIsoCode=XG. Accessed August 15, 2025. 7. FDA.gov. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRL/rl.cfm?lid=516262&lpcd=NJT. Accessed August 15, 2025.
References: 1. FPO 2. FPO 3. FPO